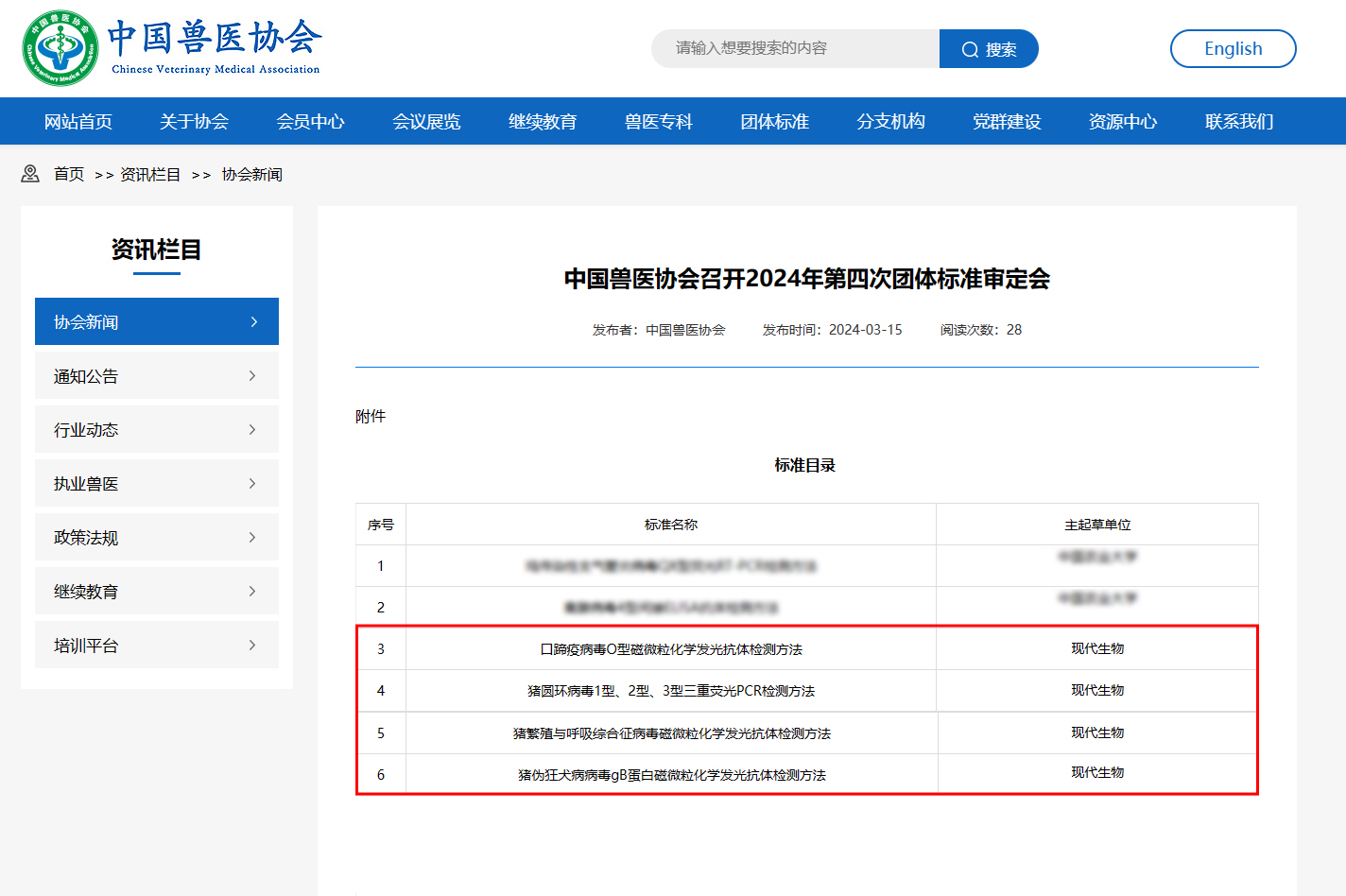
The four group standards approved this time have clinically established diagnostic methods for accurate detection of a single pathogen or rapid detection of multiple pathogens as well as specific and efficient detection of antibodies, providing technology for laboratory diagnosis, disease monitoring and epidemiological investigation of animal diseases. support.
Four group standards
"Method for detection of foot-and-mouth disease virus type O magnetic particle chemiluminescent antibody"
"Triple Fluorescent PCR Detection Method for Porcine Circovirus Type 1, Type 2, and Type 3"
"Method for Detection of Porcine Reproductive and Respiratory Syndrome Virus Magnetic Particle Chemiluminescence Antibody"
"Detection method for porcine pseudorabies virus gB protein magnetic particle chemiluminescence antibody"
Modern Biotech adheres to the concept of innovation-led development, and has successively established a large R&D team with more than 20 national scientific research institutes, including the China Center for Animal Disease Control and Prevention, China Agricultural University, Jiangnan University, Huazhong Agricultural University, and Northeast Agricultural University. Modern Biotechnology pioneered the industrialization of chemiluminescence technology for animal disease detection, and many products and technologies have filled the gaps at home and abroad.
— END —